Clinical Trial Documentatino | Clinical trials, Clinical trials study, Contract research organization

Research2note on Twitter: "The what, when and why of research essential documents @NHSRDForum @dollyblue3 @DigitalCRN @DarkNatter @Research_Innov @WeNurses https://t.co/yPlmOQEHc6" / Twitter

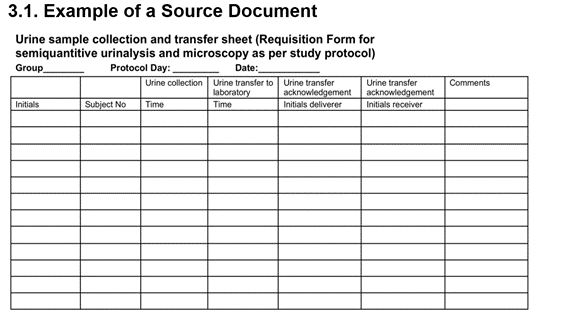
ICH GCP - 8. Essential documents for the conduct of a clinical trial: ICH E6 (R2) Good clinical practice

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health