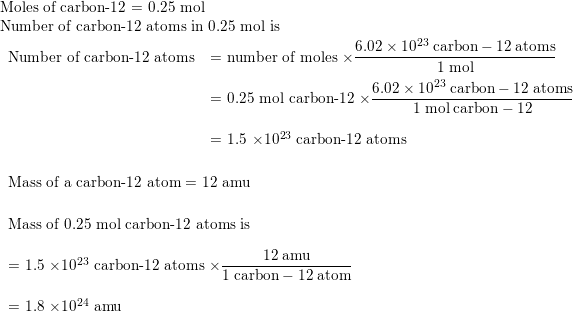SOLVED: 12.011 amu is the average atomic mass of a carbon atom. Imagine that you are able to pick up only one carbon atom from a sample, what are the chances that

SOLVED: Calculate the average atomic mass of carbon if 98.90% of the atoms are C-12 (12.000000 amu) and 1.100% are C-13 atoms (13.003354 amu).

Carbon has the following three isotopes with relative abundances and masses (amu) shown in the table. From the above date, the average atomic mass of carbon will came out to be

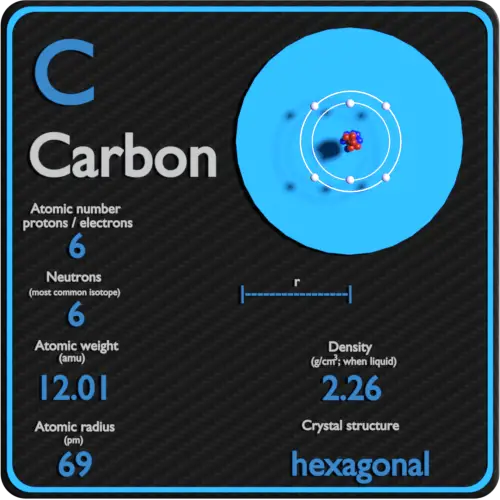
Atomic Mass Unit: amu (atomic mass unit) amu is defined as a mass exactly equal to on-twelfth the mass of Carbon-12 atom amu = 1/12 of carbon-12 Hydrogen. - ppt download