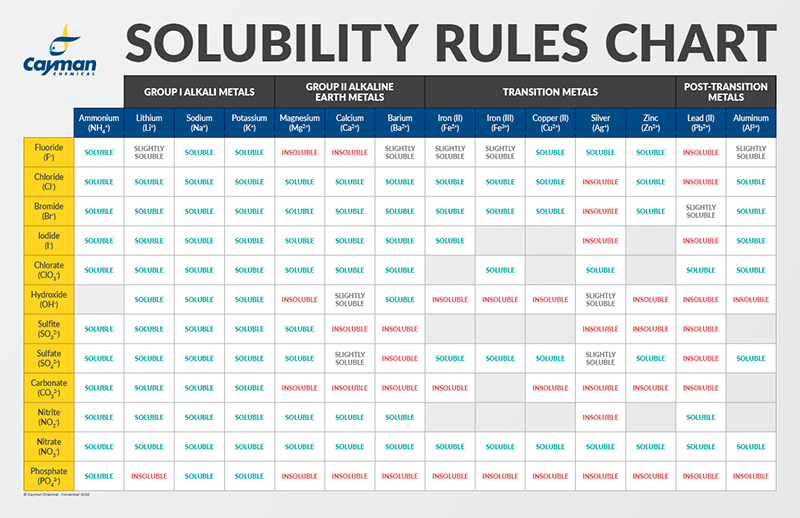
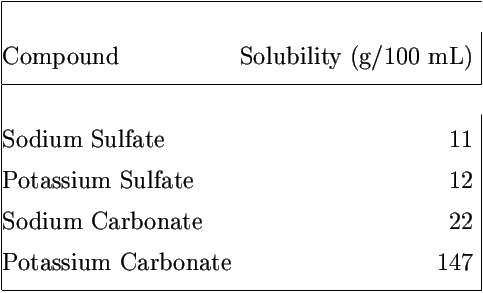
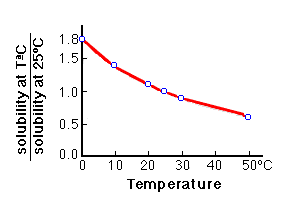
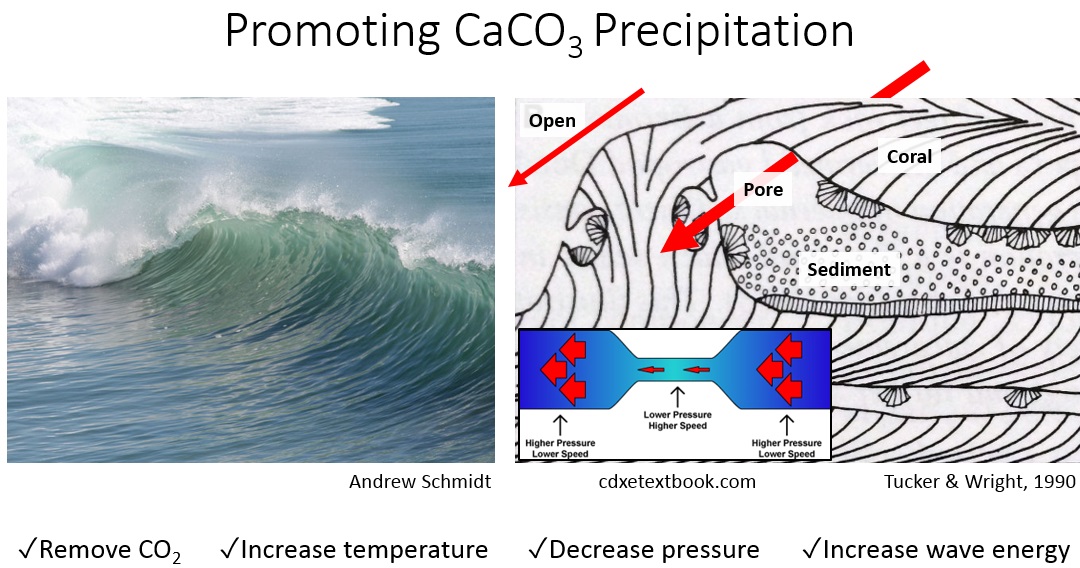
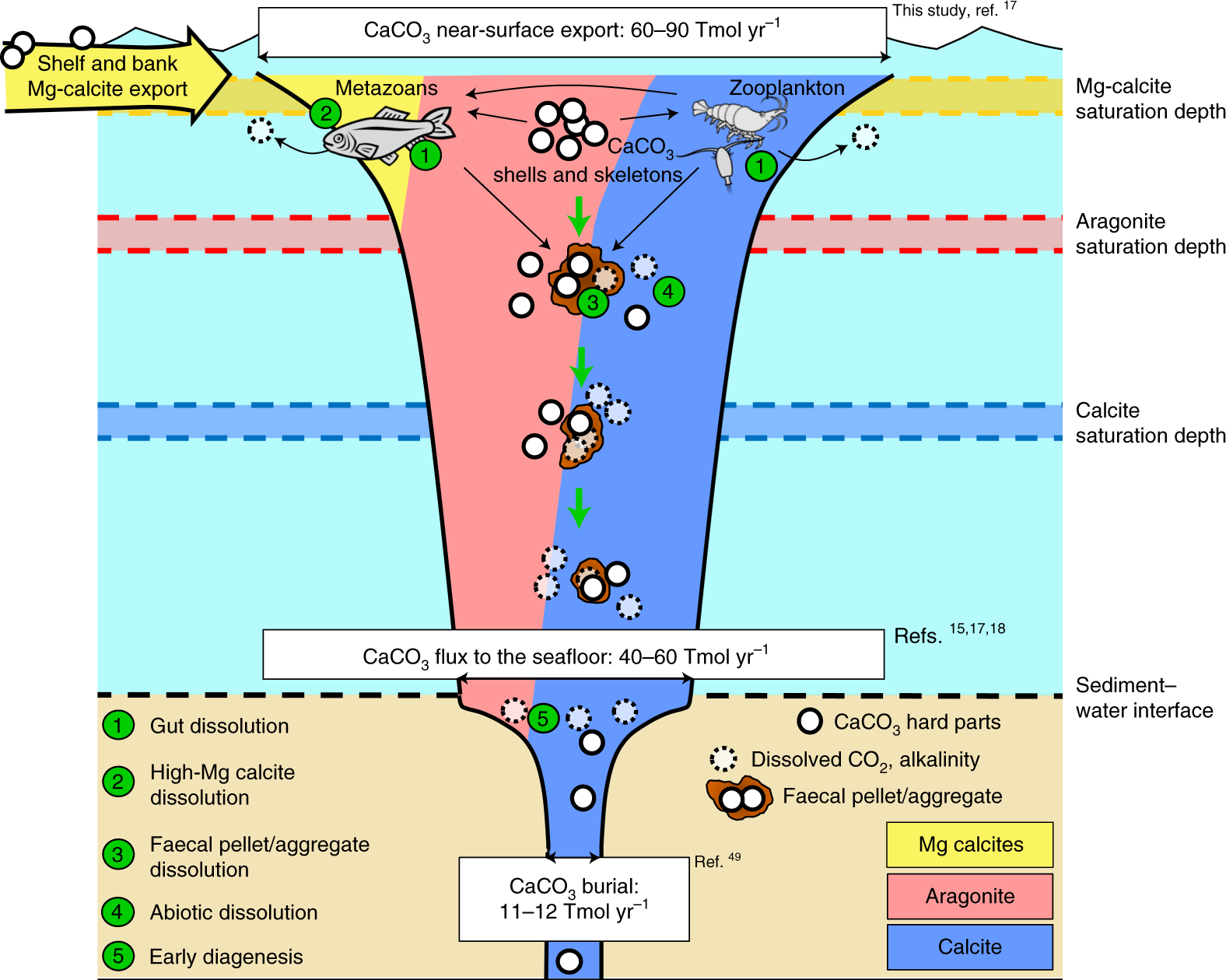
Solubility of calcium carbonate (lime scale) in water as a function of pH. | Download Scientific Diagram

inorganic chemistry - Why is sodium carbonate less soluble in water than sodium bicarbonate? - Chemistry Stack Exchange

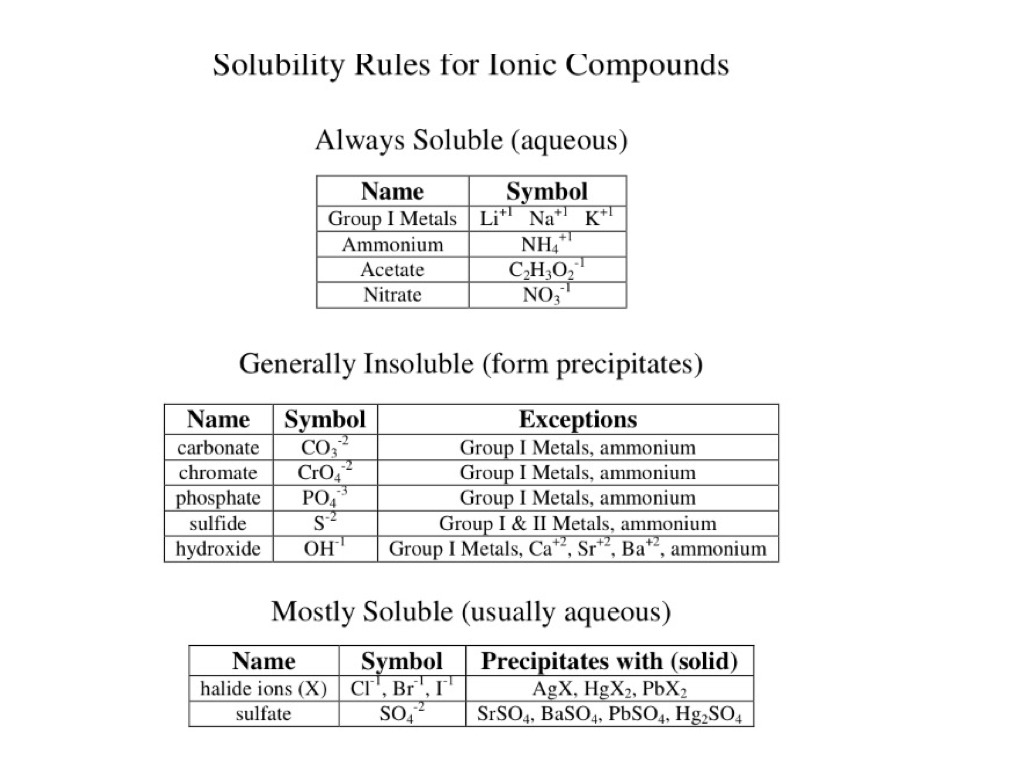
SOLVED: Identify each category of substance as soluble or insoluble in water. Most carbonate and phosphate salts blank Most halide (Br-, Cl-, and I-) salts blank Most silver salts blank salts of

![Solubility of Salts [Online Video] – O Level Secondary Chemistry Tuition Solubility of Salts [Online Video] – O Level Secondary Chemistry Tuition](https://icandochemistry942105908.files.wordpress.com/2019/02/salt-solubility.jpg?w=1200)